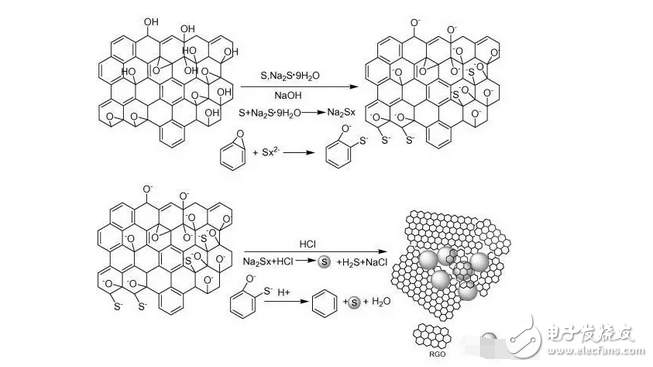
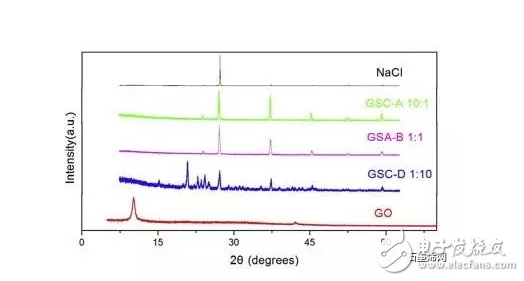
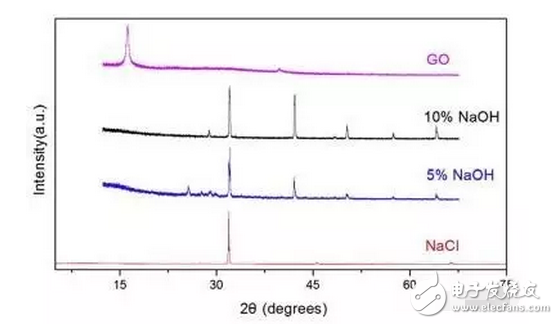
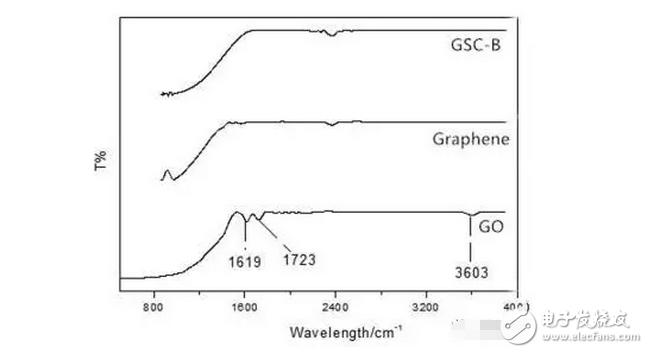
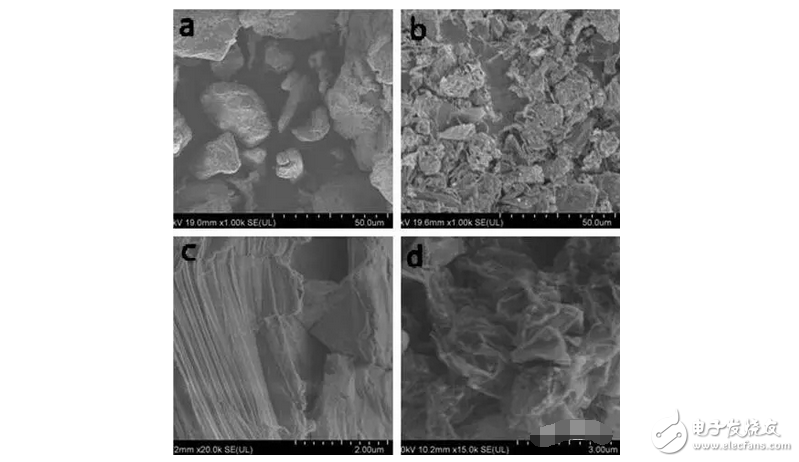
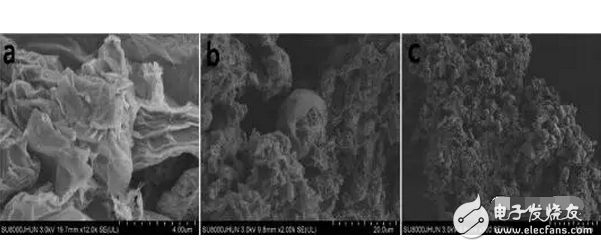
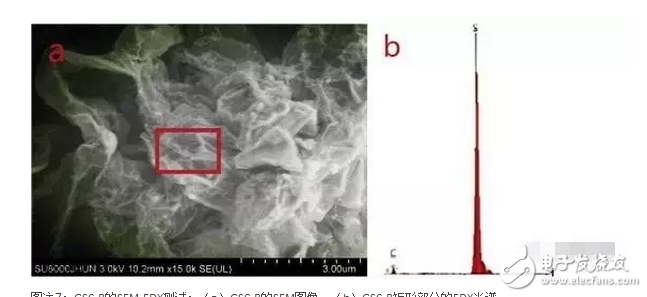
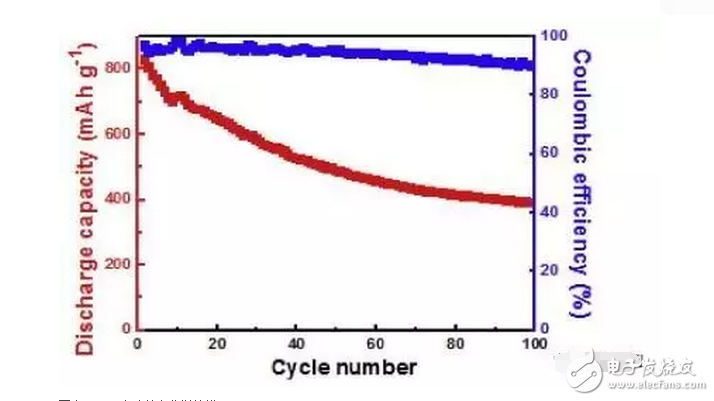
1. A sodium polysulfide solution is prepared by a reaction between sulfur and sodium sulfide nonahydrate. 2. Reduced graphene oxide (rGO) is reduced to a graphene sulfur complex (GSC) composed of graphene and sulfur. 3. This work actually paved the way for the design and manufacture of more efficient Li-S batteries. Reduced graphene oxide (rGO) coated sulfur spheres for Li-S cells were prepared by redox reaction between sodium polysulfide. The XRD spectrum shows that the diffraction peak at 10° of graphite oxide (GO) disappears, while the relatively weak diffraction peak at 27° belongs to graphene. FT-IR spectroscopy showed that the vibration of GO functional groups compared to the spectrum of GSC, such as 3603 cm-1, 1723 cm-1 and 1619 cm-1, which are derived from single bond-OH, C-O-C and C=O bonds, respectively. Disappeared. SEM observations showed that the optimal experimental conditions were: the mass ratio of GO to S was 1:1, and the pH was adjusted using 10% NaOH. EDX analysis showed that the sulfur content reached 68.8% of the composite. Under the same conditions, the resulting resistance is almost three orders of magnitude smaller than the GO resistance. Further electrochemical performance tests showed a coulombic efficiency of 96%. In 100 cycles, the first cycle capacity was 827 mAh g-1 and the first hundred times was 388 mAh g-1. This study is of great significance for the development of cathode materials for Li-S batteries. Figure 1: Schematic representation of GSC synthesis. Figure 2: XRD of NaCl, GO and GSC of different mass ratios (GO:S) Figure 3: XRD pattern of GSC synthesized under different alkaline conditions Figure 4: GSC-B, FT-IR spectrum of graphene and GO Figure 5: Scan of GO(a,c) and GSC-B(b,d) Figure 6: Scan of GSC-B (a), GSC-C (b) and GSC-D (c) Figure 7: SEM-EDX test of GSC-B: (a) SEM image of GSC-B, (b) EDX spectrum of rectangular portion of GSC-B. Figure 8: Electrochemical performance of Li-S batteries Graphical abstract Reduced graphene oxide (rGO) coated sulfur spheres for Li-S cells were prepared by redox reaction between sodium polysulfide. Under the same conditions, the resulting resistance is almost three orders of magnitude smaller than the GO resistance. This study is of great significance for the development of cathode materials for Li-S batteries. Wuxi Doton Power , https://www.dotonpower.com